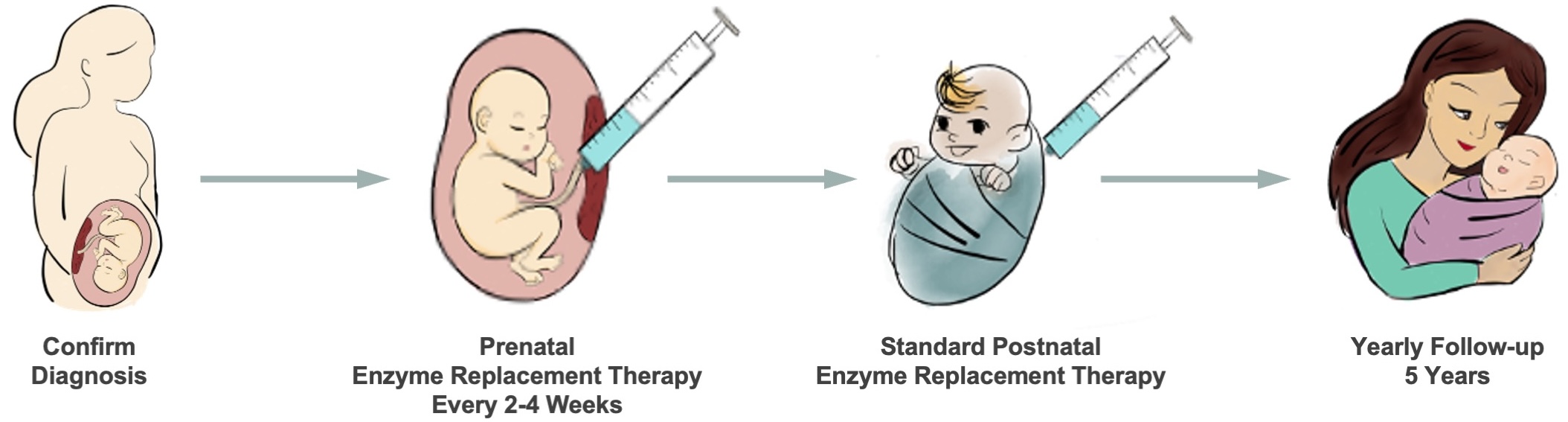
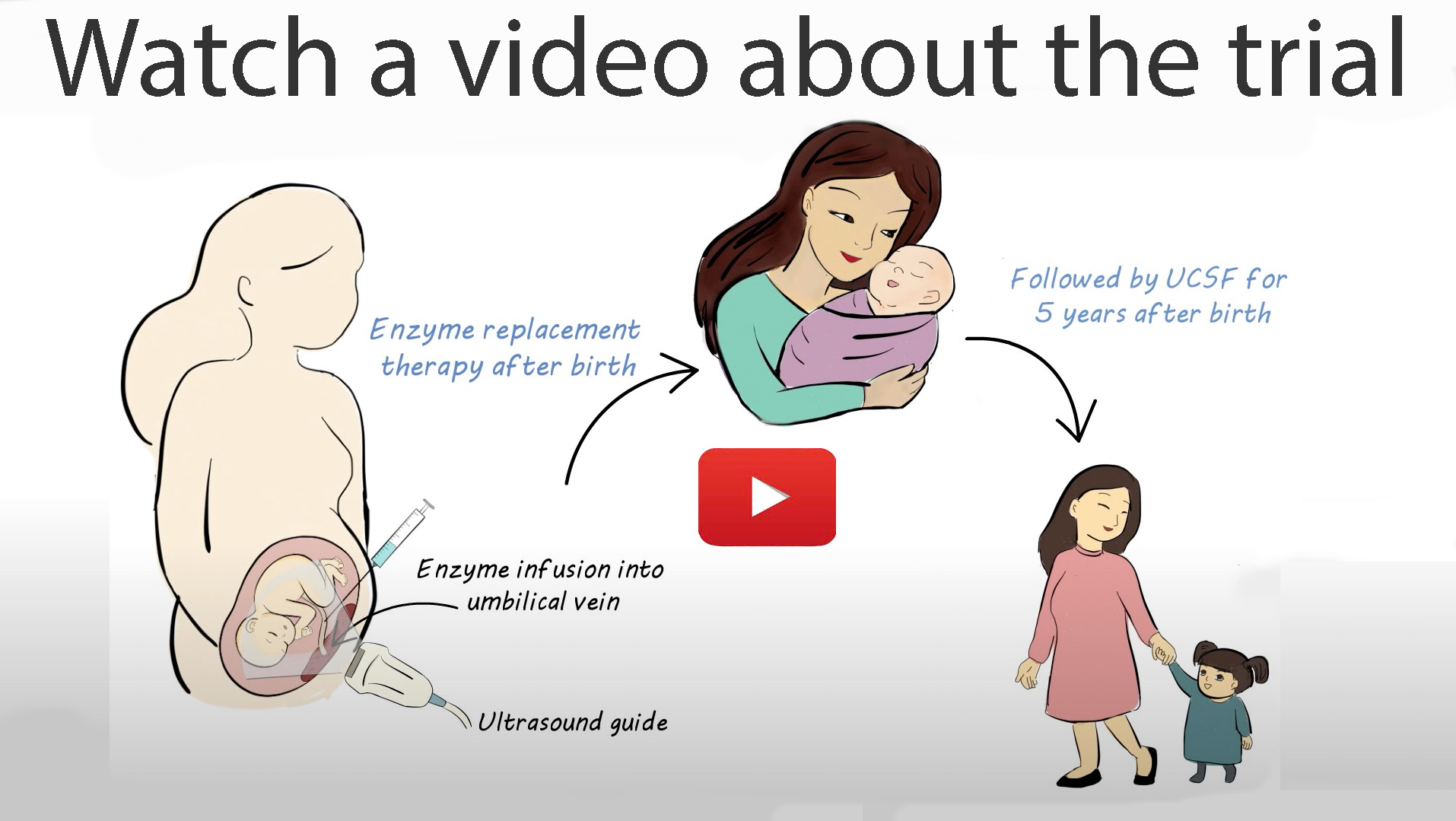
The PEARL Trial treats lysosomal storage diseases before birth. We use the same medication to treat a fetus with a lysosomal storage disease that is currently FDA-approved for children with the disease.

Earlier Intervention

UCSF Fetal Treatment Center
San Francisco, United States
Travel Support
Diseases Included in the PEARL Clinical Trial
We are testing the safety and feasibility of prenatal enzyme replacement therapy for these lysosomal storage diseases:
- Mucopolysaccharidosis (MPS) 1, or Hurler syndrome
- Mucopolysaccharidosis (MPS) 2, or Hunter syndrome
- Mucopolysaccharidosis (MPS) 4a, or Morquio syndrome
- Mucopolysaccharidosis (MPS) 6, or Maroteaux-Lamy syndrome
- Mucopolysaccharidosis (MPS) 7, or Sly syndrome
- Infantile-onset Pompe disease (IOPD)
- Neuronopathic Gaucher disease (types 2 and 3)
- Lysosomal Acid Lipase (LAL) deficiency, or Wolman disease
Study Timeline
This text was written and approved by Dr. Tippi MacKenzie, a pediatric and fetal surgeon, and Dr. Paul Harmatz, a pediatrician and metabolic disease expert, at the UCSF Benioff Children's Hospitals. Our approach was approved for use in a clinical trial by the US FDA in 2020, and our study is governed by UCSF's Institutional Review Board to ensure ethical and equitable treatment of participants.